Leaving Community
Are you sure you want to leave this community? Leaving the community will revoke any permissions you have been granted in this community.
Vasculature Morphogenesis: Synopsis of three related articles by Halina Witkiewicz, Phil Oh and Jan Schnitzer
Vasculature Morphogenesis: Synopsis of three related articles by Halina Witkiewicz, Phil Oh and Jan Schnitzer
The following is is a guest blog by Krystyna Gutowska in collaboration with the author of the articles, Dr. Witkiewicz.
*All Figures can be viewed at https://openi.nlm.nih.gov/gridquery.php?q=Halina%20Witkiewicz
The topic is a set of three articles by Witkiewicz et al. previously blogged about by NIF as an exemplar of open access publishing and a new open review process utilized by the Faculty of 1000 journals.
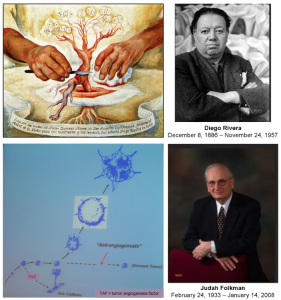
Common sense dictates that inhibiting malignant tumor growth (cancer) should be possible by inhibiting formation of new blood vessels. An artistic vision of that belief was painted in 1940 by Diego Rivera “The Hands of Dr Moore” (San Diego Museum of Art in San Diego). In 1972 Judah Folkman presented the concept for therapy of solid tumors by the anti-agiogenesis treatment [Greek angeion = vessel] for which he became well known. Yet, the quest for such treatment has not resulted in finding the cure for cancer, so far.
In countless review articles the formation of tumor vessels was presented graphically to reflect researcher’s current understanding of how it was supposed to happen at the cellular and molecular level. Instead of such artistic and graphic representations, the three studies published in F1000Research on January 10th 2013 provide photographic documentation of the process that turned out to be different from previously imagined. Nobody had expected blood elements to be produced at the site of new tissue growth first and subsequently single cells to assemble around them into vessels. That would have been counter-intuitive; after all, to handle any fluid one needs a vessel. Besides, the blood formation after birth was supposed to be localized in bone marrow (medulla ossea), not extramedullary. Therefore the new vessels were assumed to be formed first and filled with blood next. That misconception prevailed in science for a long time. The essential contributing factor was the false belief on how the red blood elements were formed. Specifically, how the erythrocyte precursors (erythroblasts) were eliminating their nuclei to become anucleated erythrocytes, meaning: red cells without nucleus.
Paradoxically, the concept of the nucleus being separated from cytoplasm by expulsion (resembling separation of egg yolk from the white) came from 1967 morphological studies that used electron microscopy, just like the 2013 studies discussed here. Although the same methodology was used at both times, the animal models subjected to the ultrastructural analysis were different. The critical difference was the type of analyzed tissue (bone marrow or spleen versus tumor). In the bone marrow and spleen the erythrogenesis is relatively rare and to observe it the process had to be experimentally stimulated by inducing anemia in dogs or mice. However, the tumor-hosting mice used recently were not subjected to any pathogenic treatment except the surgical implantation of cultured tumor cells into dorsal skin fold of the animals. In that model the erythrogenesis was spontaneous and frequent enough for capturing images of various stages. The expulsion of the nucleus from the erythroblasts was not happening and macrophages (the cells said to internalize and digest the released nuclei) were rare in that environment. The nuclei were degraded in the process of erythrogenic autophagy not by the macrophages but by the very cells they were a part of. Each erythrogenic cell was remodeled into a few smaller red vacuoles by a nucleo-cytoplasmic conversion. No nuclear waste was released for macrophages to clean up. Such red vacuoles are no longer cells, although they have been referred to as the erythrocytes (red cells). The 2013 studies use the term erythrosome (red body) as a synonym for erythrocyte because it describes the sub-cellular nature of those blood elements better. The cells converting into erythrosomes were visually recognizable in tissue sections and their location outside bone marrow was evident.
The erythrogenesis turned out to be the central element of the vasculature formation induced locally by the growing tumor or by healing of the surgical injury inflicted by the implantation of the tumor cells; figuratively speaking and literally, i.e., as a source of energy and as a structural morphogenetic element chemotacticly attracting cells. (Erythrocytes are known to secrete high energy molecules, ATP). Those ultrastructural studies demonstrated for the first time that the extramedullar hematopoiesis and vasculogenesis were inseparable in live animals; hence, the term ‘vasculature’ included blood and vessels. Historically, the term 'angiogenesis' (expansion of existing vessels) was replaced by ‘neovasculogenesis' (generation of new vessels from bone marrow derived precursor cells). Now, the most appropriate term appears to be 'vasculature morphogenesis' (formation of blood and vessels from local tissue stem cells).
In the first article the authors discuss the implications of the new findings for malignant as well as non-malignant tissue or organ morphogenesis (the organoblasts concept) and for tissue definition. The second article shows formation of a capsular vasomimicry that could potentially lead to spreading of tumor cells to various locations by fusing with morphologically similar lymphatic vessels or veins, i.e. to metastasis. The third article deals with tumor energy metabolism and explains the over half a century old Otto Warburg’s conjecture by cellular heterogeneity of tumors. That connection was missed by metabolic studies based on samples isolated from tissues because the isolation process destroys the tissue. Also missed was the role for the anaerobic metabolic pathway discovered by Warburg during cell division, when the structural changes in the mitochondria indicate their temporary functional disability. That reversible malfunction correlates with a particular stage of the cell cycle (mitosis).
The 1967 conclusion on the mechanism of the erythrocytic enucleation by expulsion of the nucleus derived form pathologically changed systems was erroneously extrapolated to systems not modified experimentally. Consequently it had a long-lasting and misleading effect on multiple studies in vascular biology. The appealing concept of inhibiting angiogenesis to stop tumor growth suffered from lack of understanding the cellular interactions leading to the vasculature formation (blood as well as vessels). To study those interactions the tissue must be preserved. Electron microscopy supported by immunocytochemistry (using antibodies to identify specific molecules in situ) is the method of choice for such purpose. The observations reported in the three articles discussed here appear of profound significance for tissue morphogenesis in general, not only in malignancy.
Refocusing on inhibiting tumor vasculature formation, with the full force of currently available technologies, presents a realistic chance to cure solid tumors in large number of patients despite the tumors’ genetic diversity perpetually introduced during cell divisions. Such strategy would not interfere with proliferation of normal cells that does not result in tissue growth. One prominent example is renewal of the gut epithelium. From clinical point of view the gut epithelium is critically important because of the role it plays in absorption of nutrients.